Hematopoietic Cell Therapy
Hematopoietic stem cells (HSCs) have long been used to provide life-saving treatments for patients with malignant and non-malignant hematological disorders. While these treatments originally took the form of conventional bone marrow transplants, more recent breakthroughs in the field have provided cell therapy researchers with methods to correct genetic mutations in a patientãs own cells for autologous HSC transplantation.
We have compiled a selection of scientific resources to help you on your way in the complex and exciting field of hematopoietic cell therapy.
CRISPR-Cas9 Editing of Hematopoietic Stem and Progenitor Cells
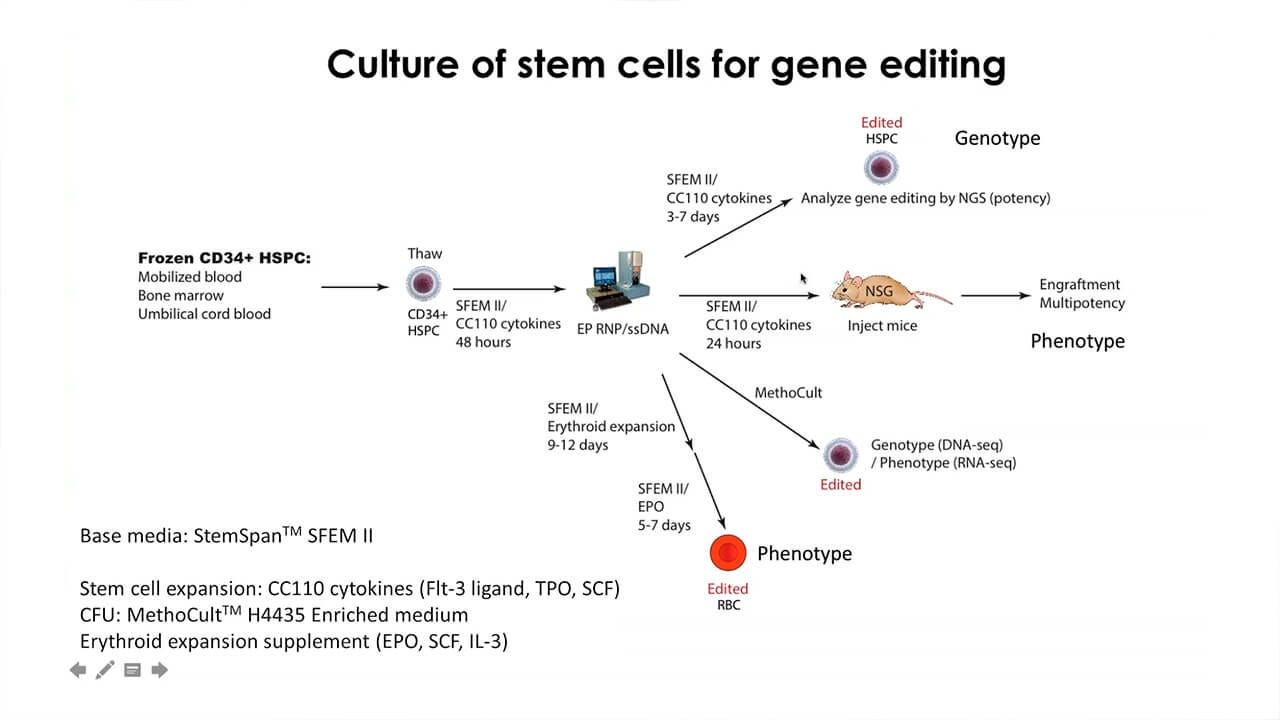
In this webinar, Dr. Mark DeWitt will discuss the use of gene editing techniques for hematopoietic stem and progenitor cells HSPCs. Topics he will include are:
- Delivery of gene editing machinery to target cells
- Homology-directed repair for precise editing
- Culture conditions required for efficient editing
- Levels of correction needed for the treatment of monogenic disorders, such as sickle cell disease
-
 The CFU Assay: In Vitro Functional Potency Assessment for Hematopoietic Stem and Progenitor CellsThe colony-forming unit (CFU) assay remains the only in vitro assay that provides a correlative assessment of hematopoietic stem and progenitor cell (HSPC) engraftment potential in vivo. For many years, regulatory bodies have recommended functional assessment and the CFU assay for labs working with cellular therapies or transplantable products derived from HSPCs. Recent updates from regulatory bodies such as FACT underscore the crucial role of the CFU assay in these workflows, particularly after any processing step, such as cryopreservation, that may impact the functional properties of HSPC products. <br><br> In this webinar, Selena Hallahan introduces the utility of the CFU assay for functional assessment of hematopoietic cell products and ¤Öêü°å¿üã comprehensive portfolio of reagents, training, and services designed to support high-performing and standardized workflows for hematopoietic cell therapy products.
The CFU Assay: In Vitro Functional Potency Assessment for Hematopoietic Stem and Progenitor CellsThe colony-forming unit (CFU) assay remains the only in vitro assay that provides a correlative assessment of hematopoietic stem and progenitor cell (HSPC) engraftment potential in vivo. For many years, regulatory bodies have recommended functional assessment and the CFU assay for labs working with cellular therapies or transplantable products derived from HSPCs. Recent updates from regulatory bodies such as FACT underscore the crucial role of the CFU assay in these workflows, particularly after any processing step, such as cryopreservation, that may impact the functional properties of HSPC products. <br><br> In this webinar, Selena Hallahan introduces the utility of the CFU assay for functional assessment of hematopoietic cell products and ¤Öêü°å¿üã comprehensive portfolio of reagents, training, and services designed to support high-performing and standardized workflows for hematopoietic cell therapy products. -
 Hematopoietic Stem Cell Fitness and Function During Sickle Cell DiseaseChronic insults, such as inflammation and replicative stress, impair and exhaust blood-sustaining hematopoietic stem cells (HSCs), leading to dysfunction and selection for leukemia-associated mutations. Dr. McKinney-Freemanãs laboratory is currently studying how sickle cell disease (SCD), an inherited hemolytic anemia with a large inflammatory component and increased hematopoietic demand, compromises the fidelity and function of hematopoietic stem cells (HSCs) in both mice and individuals with SCD. Mounting evidence indicates that SCD patients may experience enhanced rates of clonal hematopoiesis, as well as MDS (myelodysplastic syndrome) and AML (acute myeloid leukemia), in general, and following allogeneic HSC transplantation or autologous HSC gene therapy. Considering that these are the only curative therapies for SCD, it is important to better understand and prevent SCD-induced insults to HSCs and their microenvironment. <br><br> In this webinar, Dr. McKinney-Freeman from St. Jude Childrenãs Research Hospital describes, in detail, what her laboratory has learned about how SCD affects HSCs. Additionally, Dr. McKinney-Freeman is joined by Dr. Eric Norris, Account Executive, Cell Culture, ¤Öêü°å¿ü in the Q&A session.
Hematopoietic Stem Cell Fitness and Function During Sickle Cell DiseaseChronic insults, such as inflammation and replicative stress, impair and exhaust blood-sustaining hematopoietic stem cells (HSCs), leading to dysfunction and selection for leukemia-associated mutations. Dr. McKinney-Freemanãs laboratory is currently studying how sickle cell disease (SCD), an inherited hemolytic anemia with a large inflammatory component and increased hematopoietic demand, compromises the fidelity and function of hematopoietic stem cells (HSCs) in both mice and individuals with SCD. Mounting evidence indicates that SCD patients may experience enhanced rates of clonal hematopoiesis, as well as MDS (myelodysplastic syndrome) and AML (acute myeloid leukemia), in general, and following allogeneic HSC transplantation or autologous HSC gene therapy. Considering that these are the only curative therapies for SCD, it is important to better understand and prevent SCD-induced insults to HSCs and their microenvironment. <br><br> In this webinar, Dr. McKinney-Freeman from St. Jude Childrenãs Research Hospital describes, in detail, what her laboratory has learned about how SCD affects HSCs. Additionally, Dr. McKinney-Freeman is joined by Dr. Eric Norris, Account Executive, Cell Culture, ¤Öêü°å¿ü in the Q&A session. -
 Frequently Asked Questions on Primary CellsTechnical tip from our dedicated team of Product and Scientific Support specialists
Frequently Asked Questions on Primary CellsTechnical tip from our dedicated team of Product and Scientific Support specialists -
 Implementing the Colony-Forming Unit (CFU) Assay As a Potency Assay for Hematopoietic Cell Therapy ProductsAssessing the functional potency of cells for use in cell therapy research presents unique challenges. For hematopoietic stem and progenitor cells (HSPCs) in particular, potency can be measured in vitro by assessing the ability of these cells to differentiate into progenitor cells using the colony-forming unit (CFU) assay. Validating the CFU assay as a potency assay requires demonstrating its specificity, accuracy, precision, linearity, and reproducibility. Once validated, the CFU assay can assess the quality and consistency of prospective hematopoietic cell therapy products (HCTPs) at multiple stages of the processing and manufacturing workflow. <br><br> Join Dr. Colin Hammond and learn about the regulatory guidance around the potency testing of HCTPs and how to validate the CFU assay as a potency assay that can be integrated into cell therapy manufacturing workflows.
Implementing the Colony-Forming Unit (CFU) Assay As a Potency Assay for Hematopoietic Cell Therapy ProductsAssessing the functional potency of cells for use in cell therapy research presents unique challenges. For hematopoietic stem and progenitor cells (HSPCs) in particular, potency can be measured in vitro by assessing the ability of these cells to differentiate into progenitor cells using the colony-forming unit (CFU) assay. Validating the CFU assay as a potency assay requires demonstrating its specificity, accuracy, precision, linearity, and reproducibility. Once validated, the CFU assay can assess the quality and consistency of prospective hematopoietic cell therapy products (HCTPs) at multiple stages of the processing and manufacturing workflow. <br><br> Join Dr. Colin Hammond and learn about the regulatory guidance around the potency testing of HCTPs and how to validate the CFU assay as a potency assay that can be integrated into cell therapy manufacturing workflows. -
 Tools for Optimizing Human Immune Cell ResearchObtaining consistent and reliable results when culturing immune cells can be challenging. Watch this webinar to discover how to obtain high yields of functional T cells, NK cells, B cells, dendritic cells, and macrophages for your research applications. The speaker, Evan Karas, also explains how to expand primary T cells without feeders or serum.
Tools for Optimizing Human Immune Cell ResearchObtaining consistent and reliable results when culturing immune cells can be challenging. Watch this webinar to discover how to obtain high yields of functional T cells, NK cells, B cells, dendritic cells, and macrophages for your research applications. The speaker, Evan Karas, also explains how to expand primary T cells without feeders or serum. -
 Lost in Translation - Moving Your Research to Clinical TrialsThis webinar describes steps and considerations involved in translating research to the clinic.
Lost in Translation - Moving Your Research to Clinical TrialsThis webinar describes steps and considerations involved in translating research to the clinic. -
 Determining the Potency & Stability of Hematopoietic CellsIn this webinar, Dr. Jackie Damen of ¤Öêü°å¿ü describes the effects of cryopreservation protocols on the engraftment potential of hematopoietic cells and the need for standardized potency assays, including the Colony-Forming Unit (CFU) assay, for hematopoietic cell products.
Determining the Potency & Stability of Hematopoietic CellsIn this webinar, Dr. Jackie Damen of ¤Öêü°å¿ü describes the effects of cryopreservation protocols on the engraftment potential of hematopoietic cells and the need for standardized potency assays, including the Colony-Forming Unit (CFU) assay, for hematopoietic cell products.