Reliable Reagents for Revolutionary Therapies
As cell therapy development accelerates, keeping pace with the latest innovations is vital for driving your progress in research and clinical translation. Come by booth #501 at ISCT from May 7 - 10 or booth #1514 at ASGCT from May 13 - 17 to explore how our standardized and scalable solutions can help advance your research to the clinic. Weãll also be hosting talks and presenting scientific posters at both meetings.
Explore the content below for more details on our exhibitions, as well as additional scientific resources, on-demand webinars, and innovative products to support hematopoietic stem and progenitor cell culture, immune cell activation and expansion, and pluripotent stem cell differentiation for cell therapy development.
Global Showcases and Posters
Learn How We Can Support Your Regulatory and Compliance Needs
Get expert support to use our products and other ancillary materials (raw materials) for cell therapy development under the right regulatory framework. Our Services for Cell Therapy team can help you navigate complex regulatory requirements to find the best solution.
Browse Scientific Posters
Find out what ¤Öêü°å¿ü scientists have been working on.
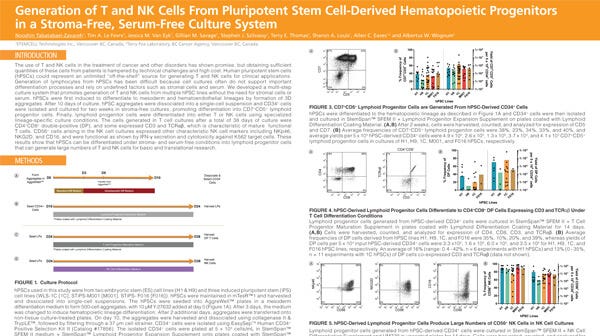
Jessie Yu, PhD, Senior Scientist
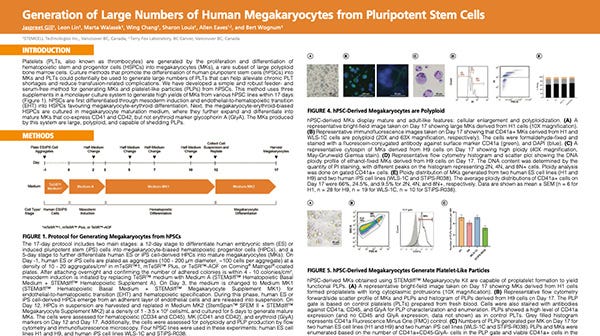
Jaspreet Gill, Research Associate
Featured Products and Solutions for Cell Therapy Development
Explore new technologies that can help you efficiently complete your experiments and take your research further.
StemSpanãÂ-AOF, GMP
Culture and expand human hematopoietic cells in animal origin-free conditions with this medium, manufactured under relevant cGMPs.
Related Webinar:
Removing Animal Components from Your HSC Workflow
MethoCultã Optimum
Achieve optimal growth of hematopoietic progenitor cells in colony-forming unit (CFU) assays of human bone marrow, mobilized peripheral blood, peripheral blood, and cord blood samples.
Related Webinar:
New Tools for the Ex Vivo Expansion of Human Hematopoietic Stem and Progenitor Cells
°Ï¯íñÀýîÝ¿ƒÝý¾ƒÝÇúýåãÂ
Automate and standardize your CFU assays. The new 21 CFR Part 11 Compliance Software Add-On supports workflows consistent with FDA Title 21 CFR Part 11 regulations on electronic records.
Related Webinar:
Implementing the Colony-Forming Unit (CFU) Assay As a Potency Assay for Hematopoietic Cell Therapy Products
Featured: TeSRãÂ-AOF, GMP
Consistently culture viral-safe, high-quality PSCs for further use in cell therapy development with an animal-origin free medium.
Related Webinar:
Quality by Design: Reagents and Support for hPSC-Derived Cell and Gene Therapies
mTeSRã Plus, GMP
Gain control over your feeding schedule and achieve superior culture morphology with this stabilized feeder-free medium for human ES and iPS cells.
Related Webinar:
How to Transition hPSCs into mTeSRã Plus
New:TeSRãÂ-AOF 3D
Rapidly scale up high-quality hPSCs without requiring adaptation from 2D culture with animal origin-free TeSRãÂ-AOF 3D.
Related Webinar
Robust Workflows for the Expansion of PSCs in 3D Suspension Culture
Featured: ݾ°ƒ°ƒ°ÉýåÇú¯ð°Éݶ°ìãÂ-°ï¿µ
Expand human T cells in serum- and xeno-free culture conditions with medium manufactured under relevant GMP guidelines for high yield and viability.
Related Video:
How to Optimize Your T Cell Therapy WorkflowãWithout the Use of Serum or Feeder Cells
Featured: GMP ImmunoCultã Human T Cell Activators
Gently activate T cells for further use in cell therapy development and manufacturingãwithout the use of magnetic beads, feeder cells, or antigens.
Related Video:
CAR T Cell Manufacturing Workflow: Isolation, Activation and Expansion
STEMdiffã NK Cell Kit
Robustly differentiate hPSC-derived NK cells for developing adoptive immunotherapies as well as for researching the basic biology of these cells
Related Video:
ISSCR Innovation Showcase: Differentiating Immune Cells from Human Pluripotent Stem Cells in Feeder- and Serum-Free Cultures
MesenCultãÂ-ACF Plus Medium
Animal component-free medium for human mesenchymal stem cells.
Related Video:
MesenCultã for Mesenchymal Stromal Cell Derivation, Culture & Differentiation
STEMdiffã Blood Vessel Organoid Kit
Media and supplements for differentiation of human pluripotent stem cells to blood vessel organoids.
Related Webinar:
Modeling the Structural and Functional Features of Blood Vasculature with Blood Vessel Organoids
MyoCultã Differentiation Kit (Human)
Medium for the differentiation of human skeletal muscle progenitor cells (myoblasts) into myotubes.
Related Webinar:
3D Modeling of Skeletal Muscle for In Vitro Functional Assaying
STEMdiffã Ventricular Cardiomyocyte Differentiation Kit
A serum-free culture medium kit for differentiation of human PSCs to ventricular cardiomyocytes.
Related Video:
Scaling-Up hPSC-Derived Cardiomyocytes for Preclinical and Therapeutic Applications

Mobilized Leukopaks
Obtain high-quality, high-volume, single-donor CD34+ cells from fresh peripheral blood leukopaks, now available from donors mobilized with either granulocyte colony-stimulating factor (G-CSF), plerixafor, or a combination of both.
Related Webinar:
How to Process a Leukopak for Downstream Cell Isolation
Human CD34+ Cells
Start experiments confidently with ready-to-use, ethically sourced human CD34+ cells from cord blood, bone marrow, or mobilized peripheral blood.
Related Webinar:
Optimizing CD34+ Cell Genome Editing for Efficiency and HSPC Maintenance
Immune Cell Subsets, Frozen
Schedule delivery of pan-T cells, B cells, NK cells, and more at your convenience.
Related Video:
How To Thaw Frozen Human Primary Cells
Fresh or Frozen Leukopaks
Obtain large numbers of fresh or frozen mononuclear cells to streamline your cell-based assays with fresh or frozen leukopaks.
Related Flyer:
Streamline your workflow with ThawSTARôÛ, an automated water-free thawing system for consistent performance. Visit: www.stemcell.com/ThawSTAR
Advancing T Cell Therapies: Insights from the Translational T Cell Talks
On June 11, 2024, ¤Öêü°å¿ü hosted the half-day virtual event ãTranslational T Cell Talks: Scaling for the Futureã in collaboration with Scientist.com. The presentations from distinguished scientists and industry leaders, provided insights into the latest developments in translational T cell research and CAR T cell innovations, and concluded with a discussion on improving collaboration between industry and academia.
Rational Combination of Cancer Therapies with PD1 Axis Blockade
Looking for ways to combine new immunotherapeutic targets and modalities for your translational research? Request this free Nature Reviews Cancer and Nature Reviews Immunology Wallchart.
Which Immune Cell Are You?
Take this personality quiz to find out which immune cell youãre most like.
Catch Up on the Latest Research with the Stem Cell Podcast
Join Daylon and Arun from the Stem Cell Podcast as they discuss the latest science along with guests like Dr. Carl June, Dr. Chuck Murray, and Dr. Connie Eaves. View the latest episode in the video below, or listen to previous episodes .